eDSP is Quince’s first drug in development that leverages our AIDE technology and is composed of DSP encapsulated in autologous red blood cells for the treatment of A-T. DSP is a corticosteroid well-described for its anti-inflammatory properties, but is coupled with serious adverse effects, including potential long-term adverse effects due to adrenal suppression. eDSP is designed to maintain the efficacy of corticosteroids while reducing or eliminating the significant adverse effects associated with corticosteroid treatment.
To date, there have been more than 7,800 infusions of eDSP in approximately 425 patients (>240 with A-T) who received at least one eDSP treatment through participation in our sponsored clinical trials or expanded access programs. 188 patients have received eDSP treatment for at least 12 months — and many for several consecutive years — without toxicities well known to be associated with chronic corticosteroid use.
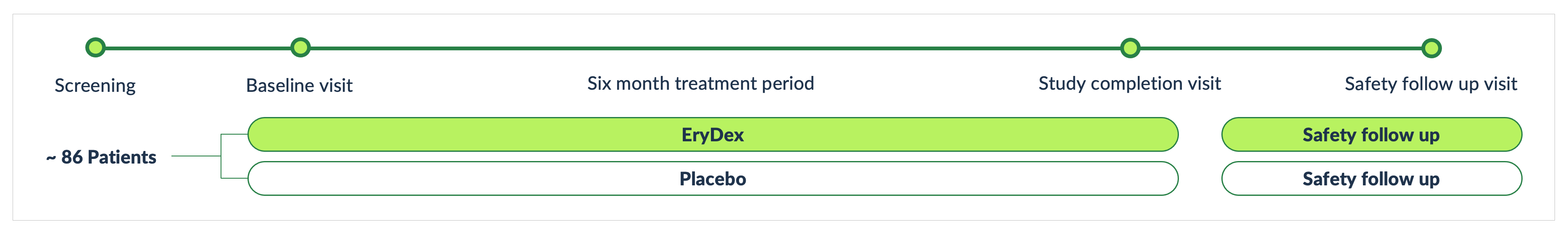
Quince is advancing a single global Phase 3 clinical trial entitled NEAT (#IDEAT-04-2022 – Neurologic Effects of eDSP on Subjects with A–T), which is a multicenter, randomized, double-blind, placebo-controlled study to evaluate the neurological effects of eDSP on patients with the rare neurodegenerative disease, Ataxia-Telangiectasia (A-T). We plan to enroll a minimum of 86 patients with A-T aged six to nine years-old and approximately 20 additional patients with A-T aged 10 years or older. This pivotal clinical trial is being conducted under a Special Protocol Assessment (SPA) that has been agreed with the U.S. Food & Drug Administration (FDA), which should allow for the submission of a New Drug Application (NDA) following completion of this single study, assuming positive results.
eDSP also has received orphan drug designation for the treatment of A-T from both the U.S. Food & Drug Administration and the European Medicines Agency. Currently, there are no approved treatments for A-T globally. The market for A-T represents a $1+ billion estimated peak sales opportunity globally.
Quince plans to investigate additional indications for eDSP where chronic corticosteroid treatment is – or has the potential to be – a standard of care. To support this expansion potential, we intend to evaluate clinical trial opportunities for potential additional rare disease indications.
Our flexible and expandable AIDE technology platform is designed to deliver a variety of therapeutics, ranging from small to large molecules, as well as biologics. Quince intends to investigate additional potential applications of the AIDE technology platform as it provides us with opportunities for future pipeline expansion.
Please contact bd@quincetx.com regarding licensing opportunities.