eDSP (previously referred to as EryDex) is Quince’s first drug in development that leverages our AIDE technology and is composed of DSP encapsulated in autologous red blood cells. DSP is a corticosteroid well-described for its anti-inflammatory properties, but is coupled with serious adverse effects, including potential long-term adverse effects due to adrenal suppression. eDSP is designed to maintain the efficacy of corticosteroids while reducing or eliminating the significant adverse effects associated with corticosteroid treatment.
To date, there have been more than 7,800 infusions of eDSP in approximately 425 patients who received at least one eDSP treatment through participation in our sponsored clinical trials or expanded access programs. 188 patients have received eDSP treatment for at least 12 months — and many for several consecutive years — without toxicities well known to be associated with chronic corticosteroid use.
Based on many years of patients being treated monthly with eDSP, eDSP may minimize the long-term serious adverse effects that are a major impediment to the chronic administration of corticosteroids. Corticosteroid therapy without significant long-term safety liabilities would represent a major advancement in the potential treatment of many chronic diseases where corticosteroids are already known to be beneficial.
eDSP was previously evaluated in multiple early-stage investigator-initiated trials and clinical trials to evaluate safety and efficacy across a number of disease indications, including our lead indication Ataxia-Telangiectasia (A-T), as well as inflammatory bowel disease, including Crohn’s disease (CD) and ulcerative colitis (UC), chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). These previous clinical studies provide a robust set of efficacy and safety data upon which our development pipeline is informed.
A recent publication in Frontiers in Drug Delivery entitled Use of Encapsulated Dexamethasone Sodium Phosphate (eDSP) in Chronic Obstructive Pulmonary Disease, Cystic Fibrosis, and Inflammatory Bowel Disorders that summarizes early-stage clinical studies of eDSP conducted across eight clinical studies in patients with pulmonary and inflammatory bowel disorders can be accessed here.
While each of these potential indications showed promising clinical study results, it was determined to advance eDSP for further evaluation of its efficacy and safety for the treatment of A-T primarily due to unmet need with no approved therapeutics for patients with A-T, previous literature showing a positive response to corticosteroids in the disease, a development path allowing for comparison to placebo control, favorable competitive landscape, and significant commercial opportunity.
Quince’s lead indication for eDSP targets a rare pediatric disease called Ataxia-Telangiectasia, or A-T. A-T is a rare inherited autosomal recessive neurodegenerative and immunodeficiency disorder caused by mutations in the ATM gene, which is responsible for cell homeostatic and cell division functions including, but not limited, to double-stranded DNA repair, cell cycle regulation, and oxidative stress response. There are currently no approved therapeutic treatments in any global market for this rare pediatric disease.
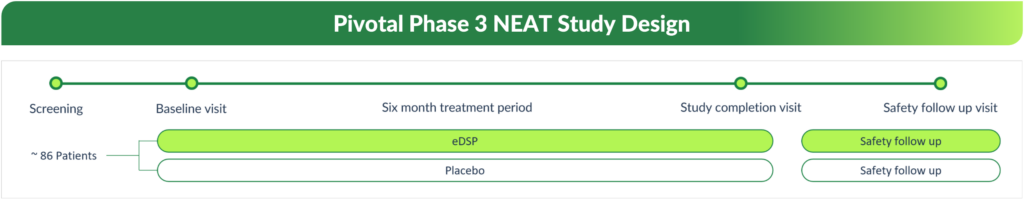
On January 29, 2026, Quince Therapeutics announced topline results from our pivotal Phase 3 NEAT clinical trial evaluating eDSP in patients with A-T.
In the NEAT study, the company’s pivotal Phase 3 international, multicenter, randomized, double-blind, placebo-controlled study (n=105), the primary endpoint, which measured the change from baseline to last efficacy visit at month six using the Rescored modified International Cooperative Ataxia Rating Scale (RmICARS) compared to placebo, did not reach statistical significance. The mean change from baseline to month six was 0.94 in the active arm compared to 2.24 in the placebo arm (difference -1.30) with a p-value of 0.0851. Furthermore, the study did not meet its key secondary endpoint of improvement in Clinical Global Impression of Severity (CGI-S) measured from baseline to month six with a p-value of 0.522.
eDSP was generally well tolerated and there were no clinically meaningful safety concerns identified. The most common adverse events reported in the eDSP arm included pruritis and pyrexia.
Learn more about the NEAT study criteria and locations by visiting Clinicaltrials.gov here and Clinical Trials Information System here.
We completed an initial patient sizing project with third-party analysis from IQVIA Medical Claims (Dx), PharmetricsPlus (P+), IQVIA Analytics, which confirmed that there are approximately 4,600 diagnosed patients with A-T in the U.S. Quince estimates that there are approximately 5,000 patients with A-T in the U.K. and EU4 countries. Currently, there are no approved treatments for A-T.
Quince intend to investigate additional potential indications for eDSP where chronic corticosteroid treatment is – or has the potential to become – the standard of care. We selected Duchenne muscular dystrophy (DMD) as our second development program for eDSP. DMD is an inherited severe muscle-wasting condition caused by X-linked recessive pattern gene mutations located on the X chromosome. The mutated dystrophin gene in DMD leads to a deficiency or absence of dystrophin protein in muscle cells. This disrupts muscle structure and function, causing progressive muscle weakness and degeneration. DMD is a progressive disease, meaning that muscle weakness worsens over time and is the most common and most severe form of muscular dystrophy diagnosed in childhood.
We consider DMD an ideal indication for eDSP as corticosteroids are the standard of care for this rare disease, but their utility is limited by significant chronic toxicity due to adrenal suppression. The standard delivery of corticosteroids by either intravenous, intramuscular, subcutaneous, oral routes for patients with DMD results in multiple peaks and troughs. Although corticosteroids can readily achieve Cmax levels required to establish efficacy, the frequent dosing repeatedly exceeds toxicity thresholds associated with adverse events, leading to debilitating serious adverse effects such as hyperglycemia, immunosuppression, and suppression of the HPA. As a result, corticosteroid treatment in patients with DMD is commonly interrupted during adolescence due to weight gain, growth suppression, cushingoid appearance, diabetes, osteoporosis, interference with sexual maturation, and delayed puberty. eDSP has the potential to provide efficacy in patients with DMD while avoiding these long-term toxicities associated with HPA axis suppression.
Quince has finalized Phase 2 clinical trial study designs to evaluate eDSP for the potential treatment of patients with DMD and plans to prioritize capital efficient study approaches, including potential investigator-initiated trials (IITs), to advance the evaluation of DMD as a second targeted eDSP indication.
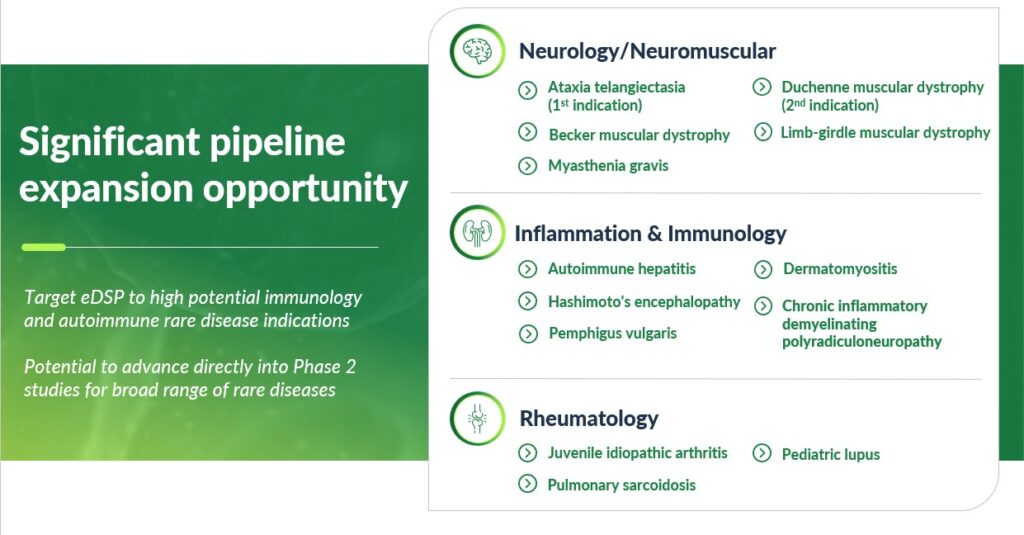
The potential for robust program expansion and new indication targeting for eDSP alone is significant. We completed an evaluation of other potential indications for eDSP beyond A-T and DMD where chronic corticosteroid treatment is or has the potential to become standard of care. This evaluation process spanned across ataxia, neuromuscular, hematology, cancer, and autoimmune disease indications with a focus on rare diseases with compelling competitive advantages and significant commercial opportunities.
We prioritized a list of other potential rare disease targets under consideration, which include: 1) autoimmune hepatitis, 2) pulmonary sarcoidosis, 3) dermatomyositis, 4) pemphigus vulgaris, 5) Hashimoto’s encephalopathy, 6) Becker muscular dystrophy, 7) pediatric lupus, 8) juvenile idiopathic arthritis, myasthenia gravis, 10) limb-girdle muscular dystrophy, and 11) chronic inflammatory demyelinating polyradiculoneuropathy.